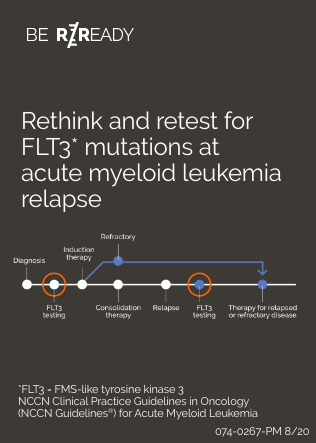
Retesting for FLT3 mutations at relapse or progression is important
Given the poor prognosis of R/R AML, you may be hesitant to wait for the results of a genetic panel.2,3 However, innovation in precision medicine has evolved such that the genetic causes of specific cancers can now be addressed through targeted therapy.4,5 That possibility now exists for the treatment of some AML subtypes, including those caused by FMS-like tyrosine kinase 3 (FLT3 ) mutations. Genetic testing is a crucial component of this paradigm shift, as identifying patients’ AML subtypes can inform possible treatment options.1 Additionally, depending on the genetic test used, results can be available in as little as 2 to 3 business days.6
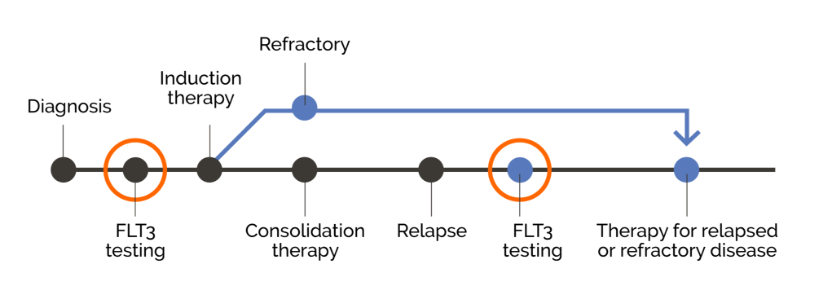
For patients with AML, confirming mutation status at relapse may open the door to potential targeted therapies1
From Bench to Bedside: The Expanding Clinical Relevance of FLT3 Mutations in the Treatment of Patients with R/R AML
Exploring how AML mutation status can inform clinical planning and the pathophysiology of AML.
FLT3 Mutations in AML: From Clinical Expression to Action
Discussing the importance of FLT3 mutations in AML, including the biological role and impact of FLT3 mutations.
Translating Science into the Clinic: The Ins and Outs of FLT3 Mutation Testing
Understanding the clinical and prognostic role of AML mutations, including when and how to assess for FLT3 mutations in patients being treated for AML.
From Bench to Bedside: The Expanding Clinical Relevance of FLT3 Mutations in the Treatment of Patients with R/R AML
Exploring how AML mutation status can inform clinical planning and the pathophysiology of AML.
FLT3 Mutations in AML: From Clinical Expression to Action
Discussing the importance of FLT3 mutations in AML, including the biological role and impact of FLT3 mutations.
Translating Science into the Clinic: The Ins and Outs of FLT3 Mutation Testing
Understanding the clinical and prognostic role of AML mutations, including when and how to assess for FLT3 mutations in patients being treated for AML.
“The field is transforming, and we are no longer treating AML the same way we did 20 years ago.”
Dr. Eunice Wang
Chief of Leukemia, Roswell Park Comprehensive Cancer Center
“The field is transforming, and we are no longer treating AML the same way we did 20 years ago.”
Dr. Eunice Wang
Chief of Leukemia, Roswell Park Comprehensive Cancer Center
Unmet need in R/R AML
FLT3 mutations are among the most common mutations in AML7
- Approximately 30% of newly diagnosed patients with AML may test positive for FLT3-ITD*3
- Approximately 7% of newly diagnosed patients with AML may test positive for FLT3-TKD**3
Patients should be retested for FLT3 mutations at relapse1 or progression, as FLT3 mutation status has been shown to change over time1,8,9,10
Changes in FLT3 Mutation Status
In a restrospective analysis of patients with relapsed AML (N=50), 22% (n=11) had different FLT3 mutation statuses at diagnosis and relapse.9
Changes in FLT3-ITD Mutation Status
In a retrospective analysis of 324 patients with AML, 83% (n=268) tested negative for FLT3-ITD mutations at diagnosis. Of those, 77 relapsed after treatment, and 10% (n=8) had a confirmed FLT3-ITD mutation at relapse.10
Changes in FLT3-TKD Mutation Status
In a retrospective analysis of 69 patients with AML, of the 60 patients with AML who tested positive for a single FLT3-ITD mutation at diagnosis, 25% (n=15) tested positive for both FLT3-ITD and FLT3-TKD after treatment.8
NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) recommend retesting all AML patients for FLT3 mutations at relapse or progression.1
Confirming FLT3 mutation status at relapse can inform an appropriate and potentially targeted treatment strategy.1
See how retesting at relapse may inform treatment options
*ITD = internal tandem duplication
**TKD = tyrosine kinase domain
- Referenced with permission from the NCCN clinical practice guidelines in oncology (NCCN Guidelines ®): for Acute Myeloid Leukemia V.3.2023. ®National Comprehensive Cancer Network. All rights reserved. Accessed April 6, 2023. To view the most recent and complete version of the guideline, go to NCCN.org. NCCN makes no warranties of any kind regarding their content, use or application and disclaims any responsibility for their action or use in any way.
- Chevallier P, Labopin M, Turlure P, et al. A new Leukemia Prognostic Scoring System for refractory/relapsed adult acute myelogeneous leukaemia patients: a GOELAMS study. Leukemia 2011;25(6):939-44.
- Patel JP, Gönen M, Figueroa ME, et al. Prognostic relevance of integrated genetic profiling in acute myeloid leukemia. N Engl J Med 2012;366(12):1079-89.
- American Cancer Society. Precision or personalized medicine (07-15-2022). https://www.cancer.org/treatment/treatments-and-side-effects/treatment-types/precision-medicine.html. Accessed 09-21-2022.
- National Cancer Institute. NCI dictionary of cancer terms: precision medicine. https://www.cancer.gov/publications/dictionaries/cancer-terms/def/precision-medicine. Accessed 01-10-2023
- Invivoscribe. LeukoStrat® CDx FLT3 mutation assay. https://catalog.invivoscribe.com/product/leukostrat-cdx-flt3-mutation-assay-2/. Accessed 10-21-2021.
- Perl AE, Altman JK, Cortes J, et al. Selective inhibition of FLT3 by gilteritinib in relapsed or refractory acute myeloid leukaemia: a multicentre, first-in-human, open-label, phase 1-2 study. Lancet Oncol 2017;18(8):1061-75. Errata in: Lancet Oncol 2017;18(12):e711.; Lancet Oncol 2018;19(7):e335; Lancet Oncol 2019;20(6):e293.
- Alvarado Y, Kantarjian HM, Luthra R, et al. Treatment with FLT3 inhibitor in patients with FLT3-mutated acute myeloid leukemia is associated with development of secondary FLT3–tyrosine kinase domain mutations. Cancer 2014;120(14):2142-9.
- McCormick SR, McCormick MJ, Grutkoski PS, et al. FLT3 mutations at diagnosis and relapse in acute myeloid leukemia: cytogenetic and pathologic correlations, including cuplike blast morphology. Arch Pathol Lab Med 2010;134(8):1143-51.
- Nazha A, Cortes J, Faderl S, et al. Activating internal tandem duplication mutations of the fms-like tyrosine kinase-3 (FLT3-ITD) at complete response and relapse in patients with acute myeloid leukemia. Haematologica 2012;97(8):1242-5.